Answer:
The correct answer is option A.
Step-by-step explanation:
Sodium hypochlorite is a strong oxidizing agent composed of 1 sodium , 1 oxygen and 1 chlorine atom.It bleaching action can be explained as due to release of nascent oxygen which oxidizes the stain and results in removal of stain from the fabrics.
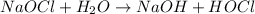
![2OCl^-\rightarrow Cl_2+2[O]](https://img.qammunity.org/2018/formulas/chemistry/college/ucpciqgl8ocx2xqyw5ojun5yj940u7etrp.png)
So, we can say that hypochlorite ion oxidizes the stain on fabric.