The volume of Carbon Monoxide gas at STP reacts to produce 759 g of Iron is 458 L.
If this is the Given:
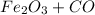
>>>
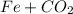
Then the first step is to balance it:
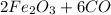
>>>

Afterwards, we convert 759 g of Iron to moles of Carbon Monoxide (CO).
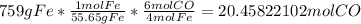
Lastly, we find the volume of carbon monoxide gas at STP, where one mole of gas occupies 22.4 Liters.
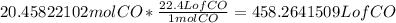
Since there are only 3 significant figures, so we round the answer to 458 L.