Answer:
Acetic acid is acting as Brønsted-Lowry acid.
Ammonia is acting as Brønsted-Lowry base.
Step-by-step explanation:
According to Brønsted-Lowry acids concept:
Acids are those chemical compounds which donates proton to other compound and forms conjugate base.
Bases are those chemical compounds which accepts proton from other compounds and form conjugate acids.
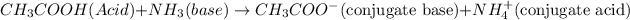
Acetic acid on donation of
 ion forms acetate ion which means it is acting as Brønsted-Lowry acid and whereas in products acetate ion
ion forms acetate ion which means it is acting as Brønsted-Lowry acid and whereas in products acetate ion
 is conjugate base.
is conjugate base.
Ammonia by accepting
 ion forms ammonium ion which means it is acting as Brønsted-Lowry base and where as in product ammonium
ion forms ammonium ion which means it is acting as Brønsted-Lowry base and where as in product ammonium
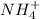 is a conjugate acid..
is a conjugate acid..