Step-by-step explanation:
Enthalpy of a solution is defined as the sum of lattice energy and hydration energy of a solution.
Symbol which represents enthalpy is
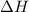 .
.
This enthalpy of a solution can have either a positive or negative value. A positive value of enthalpy means that the reaction takes place is endothermic in nature.
On the other hand, a negative value of enthalpy means that the reaction takes place is exothermic in nature.