Answer: 1 molal
Step-by-step explanation:
Molality of a solution is defined as the number of moles of solute dissolved per kg of the solvent.
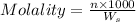
where,
n= moles of solute
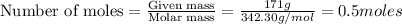
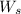 = weight of solvent in g
= weight of solvent in g
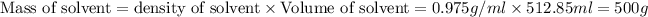
Putting in the values we get:

Thus molality of solution will be 1 molal.