The decay constant ( λ) tells us the probability of a radioactive nuclide in time. We can relate the decay constant to the half-life of the nuclide by the following equation:
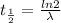
Where λ is the decay constant and t1/2 is the half-life time
We replace the value of λ and we find the half-life time
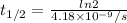
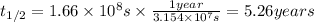
The half-time of Cobalt-60 is 5.26 years
Answer: 5.26 years