Answer : The correct option is, the number of molecules.
Explanation :
As we are given that 1 mole of oxygen gas,
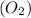 and 1 mole of carbon monoxide gas, (CO).
and 1 mole of carbon monoxide gas, (CO).
From the given options, the number of molecules must be same when comparing 1 mole of oxygen gas,
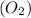 and 1 mole of carbon monoxide gas, (CO) because there are 2 molecules present in both oxygen gas and carbon monoxide gas.
and 1 mole of carbon monoxide gas, (CO) because there are 2 molecules present in both oxygen gas and carbon monoxide gas.
The mass of 1 mole of oxygen gas,
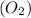 is, 32 g and the mass of 1 mole of carbon monoxide gas, (CO) is, 28 g. So, the mass will not be same.
is, 32 g and the mass of 1 mole of carbon monoxide gas, (CO) is, 28 g. So, the mass will not be same.
The number of oxygen atoms in 1 mole of oxygen gas,
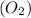 is,
is,
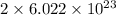 and the number of oxygen atoms in 1 mole of carbon monoxide gas, (CO) is,
and the number of oxygen atoms in 1 mole of carbon monoxide gas, (CO) is,
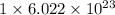 . So, the number of oxygen atoms will not be same.
. So, the number of oxygen atoms will not be same.
The volume of 1 mole of oxygen gas,
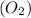 and 1 mole of carbon monoxide gas, (CO) will not be same due to the difference in the masses.
and 1 mole of carbon monoxide gas, (CO) will not be same due to the difference in the masses.
Hence, the correct option is, the number of molecules.