Answer: The correct answer is 8.
Step-by-step explanation:
Calcium is the 20th element of the periodic table. The number of electrons in this element are 20. So, the electronic configuration of this element is:
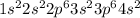
The third energy level is the level which has n = 3 and the number of electrons that are present in n = 3 are ( 2+ 6) = 8 electrons.
Hence, 8 electrons are there in the third energy level.