Answer: The correct statement is the reaction is exothermic.
Step-by-step explanation:
Enthalpy of the reaction is defined as the total sum of the energies of the products minus the total sum of the energies of the reactants. It is represented by
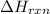 .
.
If the value of enthalpy of reaction comes out to be negative, then the given reaction is exothermic reaction and if the value of positive, the reaction is endothermic reaction.
The reaction for the formation of carbon dioxide follows the equation:
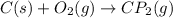
It is given that
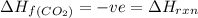
The formation of carbon dioxide is equal to the enthalpy of the reaction. As, it is negative.
Hence, it is an exothermic reaction.