Answer
(a) The nuclear reaction for bismuth-213 undergoing alpha decay is shown below:
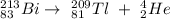
⁴₂He is the radioactive particle.
Step-by-step explanation
Alpha decay is a helium nuclei with mass number 4 and atomic number 2. So when Bismuth-213 (mass number 213, and atomic number 83) undergoes alpha decay, it means it disintegrates to emit a radioactive particle, (helium) and thallium with an atomic number of 81 and mass number 209.
Therefore, the nuclear reaction for bismuth-213 undergoing alpha decay is shown below:
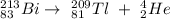
⁴₂He is the radioactive particle.