The exercise tells you that the heat (H) needed to melt copper is proportional to its mass (g).
"c" represents the number of calories needed to melt 1 gram of copper.
To determine how many calories, c, needed to melt "g" grams of copper, you have to multiply the calories per gram by the number of grams: "cg"
So the heat needed is equal to the number of calories needed to melt one gram (which is the coefficient of variation of the relationship) multiplied by the mass of copper you want to melt.
You can express this as follows:
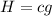
So the true statements are
B) H=cg
E) The number of calories to melt one gram is the constant of proportionality