Answer:
The correct answer is A. molarity
Step-by-step explanation:
Solutions are homogeneous mixtures (that is, their properties are uniform) of two or more substances. The substance that is found in the highest proportion is called the solvent, while the substance or substances that are in the smallest proportion are called solute.
The properties of a solution depend not only on the nature of its components but also on its concentrations.
The Molarity (M) or Molar Concentration is the number of moles of solute that are dissolved in a given volume.
The Molarity of a solution is expressed as:
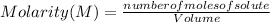
Molarity is expressed in units
 .
.