Step-by-step explanation:
It is given that pressure is 1.30 atm.
So, at STP temperature will be 273.15 K. And, according to the ideal gas equation:
PV = nRT ........ (1)
And, Density =
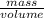
or, Volume =
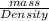 ....... (2)
....... (2)
Putting equation (2) is equation (1) we get the following.
PV = nRT
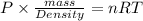
Molar mass of oxygen is 32 g/mol. Now, putting the given values into the above equation to calculate density as follows.
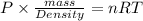
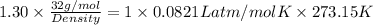
Density =
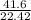 g/L
g/L
= 1.855 g/L
Thus, we can conclude that density of oxygen at 1.30 atm is 1.855 g/L.