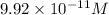Answer : The molar solubility of
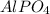 is,
is,
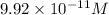
Explanation :
The balanced equilibrium reaction will be,
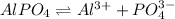
The expression for solubility constant for this reaction will be,
![K_(sp)=[Al^(3+)][PO_4^(3-)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/6hfsfebko1bclecqbvjblnz9cjz4kbg09b.png)
Let the solubility will be, 's'
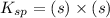
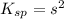
Now put the value of
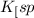 in this expression, we get the molar solubility of
in this expression, we get the molar solubility of
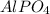 .
.
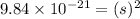
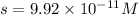
Therefore, the molar solubility of
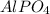 is,
is,