Answer:
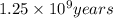
Step-by-step explanation:
The half life of potassium 40 is
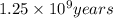 .
.
The time taken by the substance to decay into its half the quantity is called half life.
So, the time taken by the potassium 40 to decay by half amount is one half life.
Thus, the life of the rock is
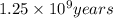 .
.