Answer: (4)electrical conductivity
Step-by-step explanation:
Given : 1.0 mole of
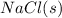 and 1.0 mole of
and 1.0 mole of
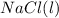
1 mole of
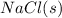 and 1.0 mole of
and 1.0 mole of
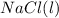 ,. both have
,. both have
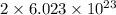 ions.
ions.
Empirical formula is the simplest chemical formula which depicts the whole number of atoms of each element present in the compound.
Thus both
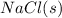 and
and
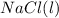 have same empirical formula.
have same empirical formula.
Both
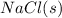 and
and
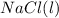 have same atoms ad thus have same gram formula mass.
have same atoms ad thus have same gram formula mass.
Electrolyte is a substance which dissociates into ions when dissolved in water and thus is able to conduct electric current through them. Compounds in solid form does not conduct electricity due to the absence of free ions.
Bus as solid compounds do not dissociate,
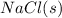 would not produce ions and thus have no electrical conductivity.
would not produce ions and thus have no electrical conductivity.
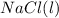 will have electrical conductivity due to presence of ions in molten form.
will have electrical conductivity due to presence of ions in molten form.