Answer:
Mass = 3.37 Kg
Step-by-step explanation:
Given the following data;
Force = 50.5N
Acceleration = 15m/s²
To find the mass of the golf ball;
Force is given by the multiplication of mass and acceleration.
Mathematically, the formula for force is;
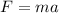
Where;
- F represents force measured in Newton.
- m represents the mass of an object measured in kilograms.
- a represents acceleration measured in meter per seconds square.
Making mass (m) the subject of formula, we have;
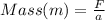
Substituting into the equation;
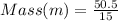
Mass = 3.37 Kg