Answer:
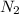 effuses at a rate that is 2.38 times that of
effuses at a rate that is 2.38 times that of
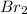
Step-by-step explanation:
We have two components:
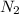 and
and
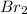 . We need to compare the rate of effusion under the same conditions.
. We need to compare the rate of effusion under the same conditions.
According to the Graham effusion law: if we have two components Component 1 (C1) and Component 2 (C2), with Molecular Weights M1 and M2 respectively. They will have a rate of effusion V1 and V2 respectively as follows:
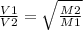
Now, we will replace for our components:
Component 1 - Nitrogen
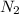
Component 2 - Bromine
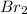
Molecular weight Nitrogen M1 = M(
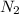 ) = 28 g/mol
) = 28 g/mol
Molecular weight Bromine M2 =M( Bromine
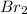 ) = 159 g/mol
) = 159 g/mol
After that, we should replace the values:

As V1 is the effusion rate of Nitrogen and V2 is the effusion rate of Bromine. We can reorganize the equation:
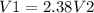
The conclusion is that V1 is 2.38 times V2.
It means
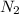 effuses at a rate that is 2.38 times that of
effuses at a rate that is 2.38 times that of
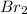 .
.