Answer : The correct option is, directly proportional
Explanation :
Formula used for lowering in freezing point :
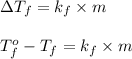
where,
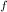 = change in freezing point
= change in freezing point
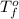 = freezing point of pure solvent
= freezing point of pure solvent
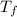 = freezing point of solution
= freezing point of solution
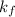 = freezing point constant
= freezing point constant
m = molality
From this we conclude that the reduction in the freezing point of a solution is directly proportional to the molal concentration.
Hence, the correct option is, directly proportional