Answer
The density of ring is 2 g/ml .
Option (A) is correct.
Explanation :
Formula
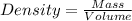
As given
Jenny accidentally drops her ring into a graduated cylinder.
If the ring has a mass of 5 grams and the water level in the cylinder rises from 5 ml to 7.5 ml .
Mass = 5 gram
Volume = Rise in volume - Initial volume
( Rise in volume = 7.5 ml , Initial volume = 5 ml)
Volume = 7.5 ml - 5 ml
= 2.5 ml
Putting the values in the formula
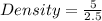
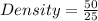
Density = 2 g / ml
Therefore the density of ring is 2 g/ml .
Option (A) is correct .