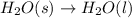Answer: Chemical changes involve the breaking of bonds in molecules.
Step-by-step explanation: Chemical change is a change in which there is rearrangement of atoms and thus there is formation of new substances which happens by making and breaking of bonds.
Example of chemical change:
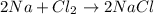
Physical change is a change in which there is no rearrangement of atoms and thus there is no formation of new substances , thus no making or breaking of bonds is involved. It only involves changes in states.
Example of physical change: