Chemistry => Stoichimetry => Limiting reactant
When they talk about the limiting reactant, they refer to the reactant that produces the least number of moles. In other words, it is the reactant that is completely consumed in the reaction.
To calculate the limiting reactant we will divide the moles given by each reactant between the respective stoichiometric coefficient.
So for calcium, we will have
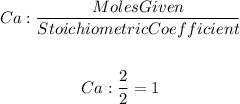
For oxygen, we will have
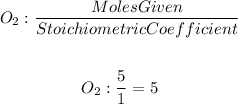
The element with the smallest quotient will be the limiting reactant. So, the limiting reactant will be calcium.
Answer: c. Calcium