Answer:The correct answer is option D.
Step-by-step explanation:
Out of these four acids the strongest acid is hydrochloric acid which completely dissociates inits aqueous solution.Where as other acids are weak acid do not dissociates complete in their aqueous solution.
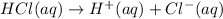
0.1 M of HCl will form 0.1 M of
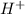 ions.
ions.
The pH is given as:
![pH=-\log[H^+]=-\log[0.1]=1](https://img.qammunity.org/2018/formulas/chemistry/high-school/ir3l32310va3mcvzua1h3acie6szoqhn0z.png)
Hence,the correct answer is option D.