Answer:
45.8 mmhg
Step-by-step explanation:
According to Dalton's law of partial pressure, the total pressure of a gas mixture is the sum of the partial pressure of the individual gases that make up the mixture.
Mathematically, the law can be expressed as:
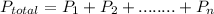
In this case,
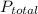 = 632.0 mmhg,
= 632.0 mmhg,
 = 124.3 mmhg
= 124.3 mmhg
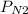 = 461.9
= 461.9
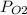 = ?
= ?
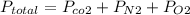
632.0 = 124.3 + 461.9 +
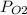
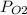 = 632.0 - + 124.3 + 461.9
= 632.0 - + 124.3 + 461.9
= 45.8 mmhg
The partial pressure of O2 is 45.8 mmhg