Answer:
The half-life of ¹³¹I is 8.04 days.
Step-by-step explanation:
We can calculate the half-life of 131I using the formula:
- 0.547 = (0.5)
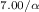
Where 0.547 is the percentage of the sample that remains, 7.00 is the amount of days that have passed, and α is the half-life.
We use Log on both sides of the equation:
- -0.262 = -0.301 * (7.00 / α)
And solve for α:
α = 8.04 days