Answer:
4.61 * 10²² oxygen atoms
Step-by-step explanation:
- Quartz is the common name of a silicon mineral, SiO₂.
- To solve the problem, we need to calculate the molar mass (MM) of quartz:
MM = AtomicMass of Si + 2 * AtomicMass of O
MM = 28.08 + 2 * 16
MM = 60.08 g/mol
- Then we can calculate how many moles of quartz are in 2.30 g:
2.30 g *
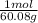 = 0.0383 mol
= 0.0383 mol
- Then we use Avogadro's number calculate the number of oxygen atoms in 0.0383 mol of SiO₂, keeping in mind the number of O per quartz molecule:
0.0383 mol SiO₂ *
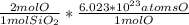 = 4.61 * 10²² atoms of O
= 4.61 * 10²² atoms of O