Step-by-step explanation:
It is known that upon losing an electron an atom tends to obtain a positive charge. Whereas upon gaining an electron an atom tends to obtain a negative charge.
Atomic number of sulfur is 16 and its electronic configuration is
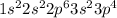 .
.
So, when a sulfur atom lose two electrons per atom then it will acquire a +2 net ion charge.