This is an incomplete question, here is a complete question.
Which of the following is the best definition of half-life for a radioactive substance?
A. Half of the amount of time required for all of the radioactive atoms to decay.
B. The amount of time required for half of the radioactive atoms to decay.
C. The amount of time required for each radioactive atom to decay halfway.
D. All of the above.
Answer : The correct option is, (B) The amount of time required for half of the radioactive atoms to decay.
Step-by-step explanation:
Half life : It is defined as the amount of time taken by a radioactive material to decay to half of its original value.
All radioactive decays follow first order kinetics.
The relation between the half-life and rate constant is:
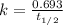
The half-life of the radioactive isotope is remains the same that means a constant value and it is not affected by any external conditions.
Hence, the correct option is, (B)