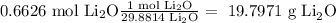Answer
19.7971 g Li₂O
Procedure
To solve this question we first need to verify if the equation is balanced. In this case, it is already balanced.
4Li(s)+O2(g)→2Li₂O(s)
Then we will need to convert the grams of both reagents to moles using the molecular weight, in order to use the stoichiometry of the reaction.
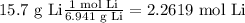
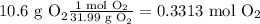
Then we determine the limiting reagent by stoichiometry as follows:
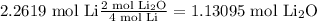
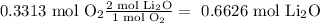
Given that the lowest value comes from molecular oxygen, this will be the limiting reagent.
Then we use the value from the molecular oxygen and convert it into grams.