Answer : The half-life of radium-230 is, 1 hr 33 min
Solution : Given,
Initial amount of radium-230 = 2.00 mg
Amount left after time, 't' = 0.25 mg
Time = 4 hr 39 min =
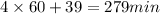 (1 hr = 60 min)
(1 hr = 60 min)
Rate law expression for first order kinetics :
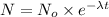
Taking 'ln' on both the sides, we get
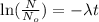
where,
N = amount left after time t
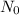 = initial amount
= initial amount
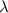 = rate constant
= rate constant
t = time
Now put all the given values in the above expression, we get
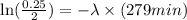
By rearranging the terms, we get
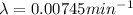
Radioactive decay follows first order kinetics.
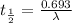
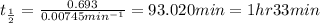
Therefore, the half-life of radium-230 is, 1 hr 33 min