Answer:
125 ml of 2.00 M solution of sulfuric acid will give 0.250 moles of sulfuric acid.
Step-by-step explanation:
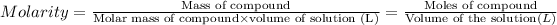
Volume of the sulfuric acid solution = V
Moles of sulfuric acid = 0.250 moles
Molarity of the sulfuric acid solution = 2.00 M
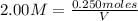
V = 0.125 L = 125 mL
125 ml of 2.00 M solution of sulfuric acid will give 0.250 moles of sulfuric acid.