Answer: The missing part in the nuclear reaction is Pb.
Step-by-step explanation:
The given nuclear reaction is a type of alpha decay process. In this process, the nucleus decays by releasing an alpha particle. The mass number of the nucleus is reduced by 4 units and atomic number is also decreased by 2 units. The particle released is a helium nucleus.
The general equation representing alpha decay process is:
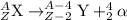
For the given equation of a nuclear reaction:
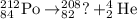
The nucleus having atomic number of 82 is Lead and the symbol used to represent this element is 'Pb'
Thus, the missing part in the nuclear reaction is Pb.