Answer:
Value of the decay constant k = 0.00012
Explanation:
Formula to determine the age of the fossils by C-14 decay is given as
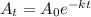
where A(t) = C-14 remaining after t years
A0 = original amount of C-14
K = decay constant
t = time taken for decay
Now we have to calculate the value of constant k when half life of C-14 is given as 5730 years.
Since half life has been given therefore final amount after 5730 years will be A/2 and initial amount will be A.
Now the equation becomes as
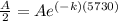
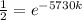
Now by taking natural log on both the sides
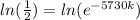
ln 1 - ln2 = -5730k (since lne = 1)
0 - ln2 = -5730k

k = 0.00012
Therefore k = 0.00012 is the answer.