Answer: The mass of
 formed will be
formed will be
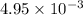 grams
grams
Step-by-step explanation:
For the given reaction:

By Stoichiometry of the reaction:
1 mole of NaCl produces 1 mole of water molecule.
So,
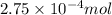 of NaCl will produce
of NaCl will produce
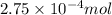 of
of

To calculate the mass of water, we will use the equation used to calculate number of moles, which is:
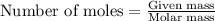
Number of moles of
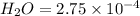 moles
moles
Molar mass of water = 18 g/mol
Putting values in above equation, we get:
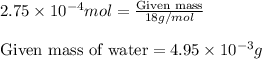
Hence, the mass of
 formed will be
formed will be
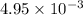 grams
grams