Answer:
167.9895 grams sample of this radioisotope would decay after a full day.
Step-by-step explanation:
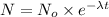
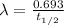
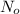 = initial amount of radioisotope
= initial amount of radioisotope
N = Amount of radioisotope left after time t.
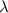 = Decay Constant
= Decay Constant
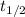 = Half life of the radioisotope
= Half life of the radioisotope
We have:
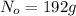
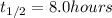
t = 1 day = 24 hour
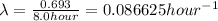
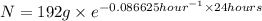
N = 24.0105 g
24.0105 grams of radioisotope will remain after 1 whole day.
Amount of radioisotope decayed =

167.9895 grams sample of this radioisotope would decay after a full day.