Anser
2 C4H10 + 13 O2 -----> 8 CO2 + 10 H2O
15 moles of H2O will be produced
Step-by-step explanation
Given:
Moles of C4H10 = 6 moles
The given unbalanced equation is
__C4H10 + __O2 --> __CO2 + __H2O
Solution:
The balanced equation for the reaction is
2 C4H10 + 13 O2 -----> 8 CO2 + 10 H2O
From the balanced equation, 4 moles of C4H10 prduced 10 moles of wH2O
Therefore 6 moles of C4H10 will produce
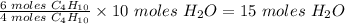
15 moles of H2O will be produced.