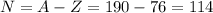Answer: 114
Step-by-step explanation:
The mass number of an element gives the sum of the protons and the neutrons inside the nucleus of one atom of that element, while the atomic number of an element gives the number of protons inside one atom of that element.
We can infer the number of neutrons inside one atom of Osmium from its mass number and atomic number.
The atomic number of osmium is 76, so each atom of osmium has 76 protons
The (average) mass number of osmium is 190, so each atom of osmium has (on average) 190 protons+neutrons
So, in order to find the average number of neutrons, we can subtract the atomic number from the mass number: