Answer:
m = 45 g
Step-by-step explanation:
mass of phosphorus added to the reaction is
m = 15 g
atomic mass of phosphorus atom is given as
W = 31 g/mol
now number of moles of phosphorus atom is given as

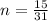
now as we can see the reaction the number of moles of phosphorus and the number of moles of the product formed must be same
So here moles of the product formed must be
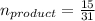
molar mass of chlorine in the product is given as
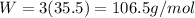
now the mass of the chlorine in product formed is given as

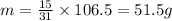
so its nearly 45 g