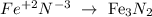There are 3 nomenclature types, so you have to be attentive of which one it is:
Hydrochloric acid: It is a hydracid, it is composed only of the elements present in the name (hydrogen and chloride).
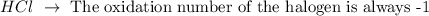
Copper (II) chloride: It's a salt composed of a metal and a halogen. The oxidation number of the halogen is -1 also in this case. This type of nomenclature tells us which is the oxidation number of the copper also, and it is the number between the parenthesis (+2).
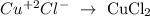
Dinitrogen pentoxide: This type of nomenclature shows the number of atoms of each element present in the formula (di is 2, and penta is 5):
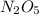
Aluminum sulfide: In the sulfides, there's the element named plus sulfur (with an oxidation number of -2). The aluminum only has +3 in its oxidation number:
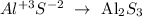
Iron (II) nitride: In this case, the nitride means it's going to be nitrogen (with an oxidation number of -3) and the other element, in this case, iron (with an oxidation number of +2, as it says in the name):