Answer:
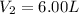
Step-by-step explanation:
Hello,
In this case, Charles' law help us to determine the required volume considering its model equation:
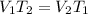
Whereas the unknown is the volume at the second state:
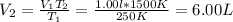
One substantiates this results considering that the higher the temperature, the greater the occupied volume by the gas.
Best regards.