Answer:
a) Temperature
b) Mass of gas
Step-by-step explanation:
The Boyles’ law states that the pressure of a gas is inversely proportional to its volume (with a fixed mass). This has been proved when temperature was kept constant.
Thus, overall there were two quantities that were not changed during Boyle’s experiment
a) Temperature
b) Mass of gas
Hence, the formula for Boyle’s law is as follows
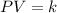
Where P is pressure
V is volume and k is constant