Answer: The correct statements are at the melting point, the entropy of a system increases abruptly as the compound transforms into a liquid, in a crystalline state, there is a tendency to minimize entropy and at absolute zero, the inter-atomic distance within a crystal is minimized.
Step-by-step explanation:
Third law of thermodynamics states that the minimum entropy must be assigned a zero value for a perfectly pure crystalline structure.
From this we can say that,
Temperature is directly proportional to the entropy of the system.
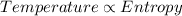
Less the temperature, less will be randomness of the particles (less will be the entropy), less will be the inter-atomic distance between the particles.
Hence, from the information above, the correct statements are:
- The entropy of a system increases abruptly as the compound transforms into a liquid,
- In a crystalline state, there is a tendency to minimize entropy
- At absolute zero, the inter-atomic distance within a crystal is minimized.