Since the container did not expand despite the change, this means that volume was constant during the process. Gay-Lussac's law states that for ideal gases with constant volume, pressure and temperature are directly proportional.
Mathematically, we have
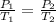
where P₁ & P₂ are the pressures and T₁ & T₂ are the temperatures of the system.
Using the values provided, we have
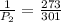
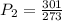
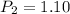
Therefore, the new pressure has a value of 1.10 atm.
Answer: 1.10 atm