The rate constant of a reaction can be computed by the ratio of the changes in the concentration and time take taken for it to decompose. Thus, if the rate constant is given to be 14 M/s, we have
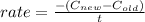
where C are the concentration values and t is the time taken for it to decompose.
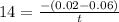
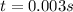
Thus, it will take 0.003 s for it to decompose.
Answer: 0.003 s