Answer:
Atomic mass is 128
Step-by-step explanation:
Atoms are composed of fundamental subatomic particles: protons, neutrons and electrons.
They are represented by a standard nuclear notation which includes the symbol of the element, its atomic number and atomic mass.
i.e.
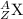
Z = atomic mass = number of protons + number of neutrons -----(1)
A = atomic number = number of protons = number of electrons----(2)
Given data:
Protons = 52
Electrons = 52
Neutrons = 76
Based on equation (1)
Atomic mass = 52 + 76 = 128