Answer : The correct option is, (1) a chemical change, because a compound is formed
Explanation :
Physical change : It is a change in which no new compound or substance is formed only changes in the phase.
Chemical change : It is a change in which a new compound or substance is formed and also some energy releases. There is no changes occurs in the phase.
When a molecule of
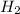 and a molecule of
and a molecule of
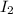 collide with proper orientation and sufficient energy then the chemical changes occurs due to the formation of the new compound.
collide with proper orientation and sufficient energy then the chemical changes occurs due to the formation of the new compound.
Hence, the correct option is, (1) a chemical change, because a compound is formed