Answer : The correct option is, No, because the carbon chain has no double bonds.
Explanation :
Aromatic hydrocarbon : It is a type of hydrocarbon in which the sigma bonds and delocalized
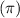 electrons are present between the carbon-carbon atoms that forming a circle.
electrons are present between the carbon-carbon atoms that forming a circle.
The given molecule is cyclohexane having the formula,
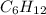
In the cyclohexane molecule, only sigma bonds are present between the carbon-carbon atoms. There is no delocalized
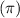 electrons are present between the carbon-carbon atoms.
electrons are present between the carbon-carbon atoms.
Hence, the given molecule is not an aromatic hydrocarbon because the carbon chain has no double bonds.