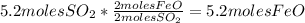Answer:
First line of the table:
FeS: 1.2mol
 : 1.8moles
: 1.8moles
FeO: 1.2moles
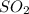 : 1.2moles
: 1.2moles
Second line of the table:
FeS: 5.2mol
 : 7.8moles
: 7.8moles
FeO: 5.2moles
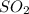 : 5.2moles
: 5.2moles
Step-by-step explanation:
1. Take as reference the balanced chemical equation:
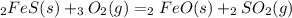
2. Take the first quantity given in the table to calculate the quantities of the other compounds using stoichiometry:
- Calculate the number of moles of
 :
:
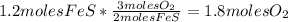
- Calculate the number of moles of FeO:
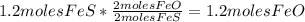
-Calculate the number of moles of
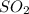 :
:
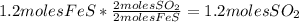
3. Take the second quantity given in the table to complete the other quantities using stoichiometry:
- Calculate the number of moles of FeS:
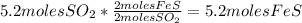
- Calculate the number of moles of
 :
:
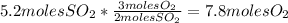
- Calculate the number of moles of FeO: