Answer: B) 20
Explanation: Atomic number is equal to the number of electrons or the number of protons for a neutral atom.
Mass number is the sum of number of protons and the number of neutrons.
Mass number = Number of protons + Number of neutrons = 47
General representation of an element is given as:
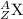 where,
where,
Z represents Atomic number
A represents Mass number
X represents the symbol of an element
Calcium has atomic number of 20. For the given isotope of calcium,
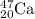
Thus number of protons = 20.