Answer: Option (b) is the correct answer.
Step-by-step explanation:
The elements which have excess or deficiency of electrons will react readily.
Atomic number of Mn is 25 and electronic configuration of
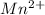 is [Ar]
is [Ar]
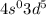 . This configuration is stable.
. This configuration is stable.
Atomic number of Cr is 24 and electronic configuration of
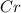 is [Ar]
is [Ar]
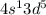 . This configuration is not stable.
. This configuration is not stable.
Atomic number of Fe is 26 and electronic configuration of
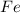 is [Ar]
is [Ar]
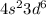 . This configuration is stable.
. This configuration is stable.
Atomic number of Cu is 29 and electronic configuration of
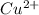 is [Ar]
is [Ar]
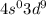 . This configuration is not stable.
. This configuration is not stable.
Atomic number of Al is 13 and electronic configuration of Al is
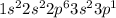 . This configuration is not stable.
. This configuration is not stable.
Atomic number of Ba is 56 and electronic configuration of
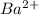 is [Kr]
is [Kr]
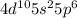 . This configuration is stable.
. This configuration is stable.
Atomic number of Mg is 12 and electronic configuration of
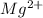 is
is
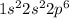 . This configuration is stable.
. This configuration is stable.
Atomic number of Sn is 50 and electronic configuration of Sn is [Kr]
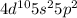 . This configuration is stable.
. This configuration is stable.
Thus, we can conclude that out of the given options, only Fe and
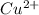 reactants would lead to a spontaneous reaction as they have incomplete sub-shells. Therefore, in order to gain stability they will readily react.
reactants would lead to a spontaneous reaction as they have incomplete sub-shells. Therefore, in order to gain stability they will readily react.